31 minutes ago
About sharing
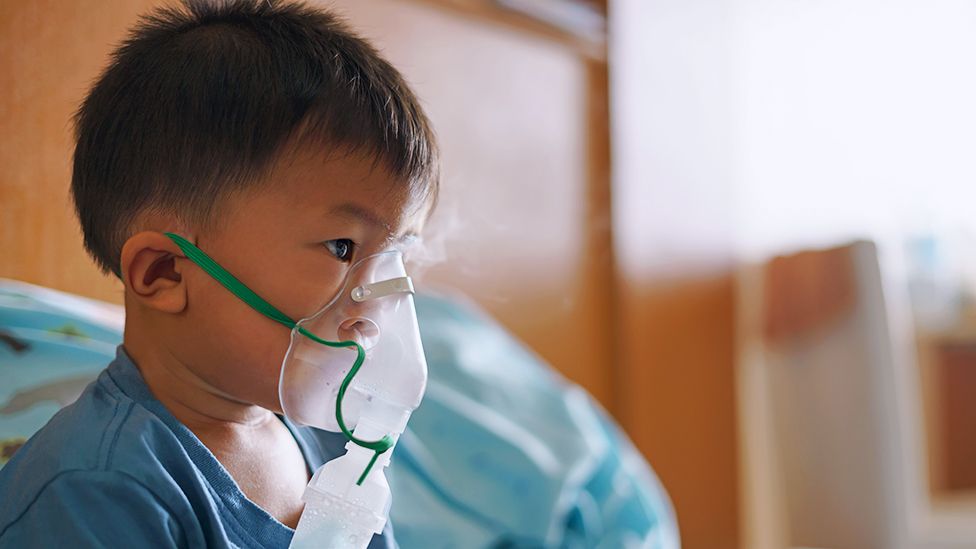
Hospital admissions from a winter virus could be reduced by more than 80% if babies are given a single dose of a new antibody treatment, a study says.
Respiratory syncytial virus (RSV) usually causes mild, cold-like symptoms, but can lead to bronchiolitis and pneumonia.
More than 30,000 under fives are hospitalised with RSV in the UK annually, resulting in 20 to 30 deaths.
One parent said her son getting RSV was “very scary” as a first-time mother.
Lorna and Russell Smith’s eldest son, Caolan, got the virus when he was eight months old and was admitted to hospital twice – each time requiring oxygen.
Now aged two, he has made a full recovery.
“I hadn’t heard of RSV and wasn’t sure what to do. He had laboured breathing due to high temperature and was quite lethargic. It brought a lot of anxiety and stress,” Lorna said.
The family, from Southampton, hope their one-month-old Rian will be able to have the RSV antibody injection if it is approved for use in the NHS.
The Harmonie study involved 8,000 children up to the age of 12 months in the UK, France and Germany, with half receiving a single dose of the monoclonal antibody treatment nirsevimab.
The results, published in the New England Journal of Medicine, showed that RSV-related hospitalisation was reduced by 83% in those receiving the jab and admissions for all chest infections were cut by 58%.
Side effects were similar in both groups and mostly mild.
Kate and Matt Parker’s twin daughters, Jessica and Ellie, took part in the trial in Southampton when they were three months old.
Kate told the BBC: “I hoped that if one of them got the jab they would have a good chance of remaining healthy over the winter season. Jess was immunised, but both were well.”
She said the trial results were “fantastic” and “if it could prevent thousands of children going into hospital and putting more strain on the NHS during the winter, that would be great.”
Nirsevimab, produced by Sanofi, was licensed for use in the UK last year.
The Joint Committee on Vaccination and Immunisation (JCVI), which advises the government, is considering whether to recommend the jab to protect infants. It is also analysing data on a RSV vaccine given to pregnant women which was licensed last month.
The JCVI says a cost-effective RSV immunisation programme should be developed for both infants and older adults.
Unlike a vaccine, which prompts the body to create antibodies and takes a few weeks to be effective, nirsevimab gives immediate protection.
Prof Saul Faust, co-study leader at the University of Southampton and a consultant paediatrician, said: “These latest results show that this long-acting antibody is safe and could protect thousands of babies from hospitalisation when used in conditions similar to routine clinical practice. It is really important information for the UK to help decide on options for the future national RSV immunisation programme.”
In July, the US Food and Drug Administration (FDA) approved nirsevimab for babies and it has also been rolled out in parts of Spain.
How to spot RSV
•RSV starts with a blocked or runny nose and can progress to a dry cough, fever and sometimes breathing problems
•For most children, it will be mild and can be treated at home with infant paracetamol or ibuprofen
•Call your GP or seek medical advice if your child is not feeding normally, is breathing fast or has a high temperature that will not go down
•Call 999 if your child is exhausted from trying to breathe – you may see the muscles under their ribs sucking in with each breath or they may be pale and sweaty
Related Topics
19 May
19 February
30 September 2022